Introduction: A Beacon of Hope Against Cancer
For decades, the word cancer has carried a weight of fear, pain, and uncertainty. Despite progress in treatments, patients undergoing chemotherapy, radiation, and surgery often face not only physical exhaustion but also long-term side effects and financial strain. Against this backdrop, the announcement of a cancer vaccine that shows 100% efficacy in early human trials feels nothing short of extraordinary.
Russia has recently unveiled Enteromix, a personalised cancer vaccine that has stunned the medical world with its initial results. Revealed at the 2025 St. Petersburg International Economic Forum, this discovery represents more than just scientific advancement; it embodies hope. For the first time, patients and families across the globe may imagine a future where cancer is not a death sentence but a disease the human immune system can outsmart.
The Science Behind Enteromix
Enteromix is based on the same messenger RNA (mRNA) technology that powered the world’s first COVID-19 vaccines. The principle is both elegant and powerful: mRNA is used to instruct the body’s immune system to recognise specific proteins—in this case, those present on cancer cells. Once trained, the immune system can identify, attack, and eliminate malignant cells.
Unlike chemotherapy, which indiscriminately targets rapidly dividing cells (including healthy ones), Enteromix is highly selective. It is also personalised: each vaccine is tailored to the unique tumour profile of the individual patient. For the initial trials, Enteromix was customised for colorectal cancer patients, one of the most prevalent and deadly forms of cancer globally.
“This is a therapy designed not just to treat cancer, but to teach the body to defeat it intelligently,” said one Russian researcher involved in the project. That makes this cancer vaccine not just a treatment but an evolving weapon that works in harmony with the patient’s own biology.
Trial Results: Safety and Efficacy
The Phase I trials enrolled 48 volunteers, all of whom had advanced colorectal cancer. Conducted by the National Medical Research Radiological Centre in collaboration with the Engelhardt Institute of Molecular Biology, the trial sought to evaluate both safety and effectiveness.
The outcomes stunned observers:
- 100% efficacy – all patients showed tumour shrinkage.
- No serious adverse reactions – unlike chemotherapy, which often brings nausea, fatigue, hair loss, and organ toxicity.
- Strong immune response – laboratory analysis confirmed that the vaccine successfully trained patients’ immune systems to recognise cancer cells.
The announcement was made publicly during the 2025 St. Petersburg International Economic Forum, positioning the discovery not only as a medical milestone but also as a matter of national pride for Russia. Regulatory approval from the Russian Ministry of Health is reportedly just one step away. If granted, Enteromix could become the world’s first approved personalised cancer vaccine.
Why This Cancer Vaccine Matters
Traditional cancer treatments save lives, but at a steep cost to patients’ quality of life. Chemotherapy and radiation can leave patients physically weakened, emotionally drained, and financially devastated.
Enteromix offers several groundbreaking advantages as a cancer vaccine:
- Precision over destruction – It targets cancer cells without harming healthy tissues.
- Personalisation – Each patient’s vaccine is tailored to their tumour’s genetic profile.
- Fewer side effects – Patients reported no severe complications during trials.
- Potential for scalability – If successful in larger trials, mRNA cancer vaccines could be adapted for multiple cancer types.
If validated in broader studies, this could represent a paradigm shift in oncology, moving from destructive treatments to intelligent, immune-based strategies. Instead of patients fearing the treatment as much as the disease, a cancer vaccine could provide a gentler yet more effective alternative.

A Cautious Optimistic reaction
The international medical community has greeted the announcement with a mix of excitement and caution. Many oncologists and researchers have emphasised that while the results are extraordinary, they come from a small-scale trial. Larger, multi-phase clinical trials are essential before Enteromix can be considered a definitive solution.
Still, experts agree that the potential is enormous. The success of COVID-19 mRNA vaccines has already proven that this platform can be rapidly developed, manufactured, and distributed on a global scale. Applying the same approach to oncology could accelerate access to lifesaving therapies, especially in the form of a cancer vaccine.
Pharmaceutical companies, biotech investors, and public health authorities worldwide are closely monitoring Russia’s progress. Some analysts even suggest that this breakthrough could trigger a new era of international collaborations or, conversely, a geopolitical race to dominate next-generation cancer vaccines.
Global Landscape and the Future of Cancer Vaccines
Russia’s Enteromix is making headlines, but it is not the only effort in the race toward a personalised cancer vaccine. Across the world, governments, researchers, and pharmaceutical companies are exploring the same frontier.
In 2023, the UK’s National Health Service (NHS) launched the Cancer Vaccine Launch Pad in collaboration with BioNTech. The programme aims to speed up access to mRNA-based personalised vaccine clinical trials for newly diagnosed patients, while accelerating development so these therapies can move from laboratories to hospitals more quickly.
The United States, meanwhile, has taken a more cautious path. To date, the Food and Drug Administration (FDA) has only approved one therapeutic cancer vaccine, Sipuleucel-T, for prostate cancer back in 2010. This personalised vaccine collected immune cells from patients, exposed them to a protein linked to prostate cancer, and reintroduced them into the body. While groundbreaking at the time, it extended survival by only four months and did not transform cancer care.
Indian oncologists stress both optimism and caution. Dr. Abhishek Shankar of AIIMS noted that while global teams are working on vaccines for multiple cancers, success has so far been limited. He also warned of affordability: “Whenever a new product is approved, the cost is likely to be very high. Then we will have to see whether there is enough mortality benefit to using it. Will the patients be cured? Will they be able to live for several years? This has to be factored in.”
Currently, more than 120 clinical trials of cancer vaccines are underway worldwide, targeting lung, breast, prostate, melanoma, pancreas, and brain cancers, according to Dr. Arvind Krishnamurthy of the Cancer Institute, WIA, Chennai.
If Enteromix clears larger trials and regulatory scrutiny, it could become the world’s first broadly successful therapeutic cancer vaccine, marking a milestone in oncology. More importantly, it could accelerate a global shift toward personalised medicine, where cancer treatments are no longer one-size-fits-all, but instead designed around each patient’s unique tumour biology.
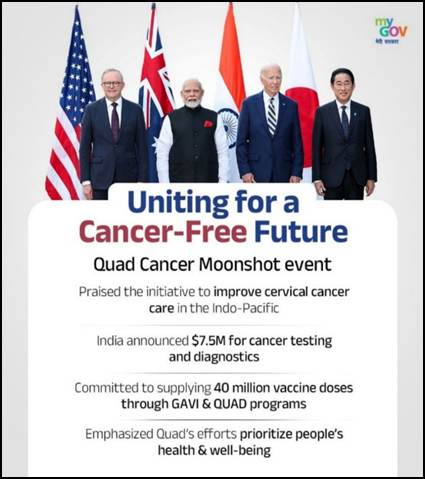
What It Could Mean for India
For India, where cancer cases are rising sharply, Enteromix could be transformative. According to the Indian Council of Medical Research, the country records over 1.4 million new cancer cases annually, with colorectal, breast, and lung cancers among the most common.
Yet, access to advanced cancer care remains uneven. Rural areas suffer from limited oncology infrastructure, and many patients struggle with high treatment costs.
A safe, effective, and personalised cancer vaccine could:
- Reduce treatment costs compared to prolonged chemotherapy cycles.
- Improve survival rates through targeted therapy.
- Expand accessibility if adapted to India’s healthcare system.
- Offer hope for rural populations who lack access to specialised cancer hospitals.
Indian oncologists are already expressing keen interest. However, much will depend on whether Russian regulatory approval leads to international recognition and whether India can secure pathways for clinical trials, importation, or local production of the cancer vaccine.
Challenges and the Road Ahead
While the early data are encouraging, Enteromix still faces several challenges before it can reach global patients:
- Larger Trials Needed – The sample size of 48 is too small to establish universal safety and efficacy. Phase II and III trials involving thousands of patients will be critical.
- Regulatory Hurdles – Approval outside Russia will require scrutiny by agencies like the U.S. FDA, European Medicines Agency, and India’s DCGI.
- Cost and Accessibility – Personalised cancer vaccines are expensive to produce. Ensuring affordability in low- and middle-income countries will be a major test.
- Long-Term Data – Researchers need to observe whether the effects are durable and whether the cancer could recur.
- Geopolitical Factors – International acceptance of a Russian-developed drug may face political and economic barriers.
Despite these obstacles, Enteromix aligns with a broader global trend toward personalised medicine. If the vaccine proves successful, it could transform oncology for generations, turning what was once a deadly disease into a manageable condition.
Conclusion: Hope with Caution
Russia’s Enteromix cancer vaccine stands as one of the most promising medical developments of the decade. A treatment that is both personalised and side-effect-free is almost unheard of in oncology, and its success has lit a spark of optimism across the globe.
For India, where access and affordability remain major barriers, such a cancer vaccine could be revolutionary. But we must temper hope with caution. Until larger trials are complete, until regulators across the world give their approval, and until affordability challenges are solved, Enteromix remains a promise, not yet a cure.
Still, the possibility is too powerful to ignore. If the results hold true, we may be witnessing the dawn of a new era—one where the words “cancer vaccine” are no longer a dream, but a reality offering millions a chance at life and hope.
- Sheelu Kumari
MUST READ: ACROSS THE BORDER: WHY NEPAL’S YOUNG VOICES ARE BEING HEARD WORLDWIDE

